ALCL and breast implants? The rare incidence of Anaplastic Large Cell Lymphoma
UPDATED 9/1/24: Here are the most recently updated facts about ALCL and textured breast implants:
Data from the American Society of Plastic Surgeons BIA-ALCL Global Network shows that there are currently 1687 known cases of BIA-ALCL and 59 deaths across 51 countries worldwide as of April 1, 2024. The last published report from the U.S. Food and Drug Administration before these data were presented, aggregated from health regulator reports in 19 countries, found 1264 BIA-ALCL cases and 63 deaths worldwide as of June of 2023.9 A survey of European countries identified a significant increase in BIA-ALCL cases reported from April of 2019 to November of 2020.10–12 Descriptive characteristics of patients with BIA-ALCL found in the case report literature are presented in Table, Supplemental Digital Content 2, https://links.lww.com/PRS/G908.
Read the article published September 2024 here.
What is breast implant associated-anaplastic large cell lymphoma (BIA-ALCL)?
- A rare type of lymphoma that develops adjacent to breast implants.
- It usually develops as a swelling of the breast 3 to 14 years after the insertion of breast implants, which is due to fluid collecting around the implant or it can present as a lump in the breast or armpit.
BIA-ALCL is not breast cancer
- BIA-ALCL develops in the fluid around breast implant and is usually contained by the fibrous capsule around implants it does not develop in the breast tissue.
What are the symptoms of BIA-ALCL?
- The most common symptom is a persistent swelling of the breast but can include other symptoms such as a lump in the breast or armpit.
- These symptoms develop between three and 14 years following the insertion of breast implants and most commonly around eight years or later.
- The swelling of the breast is due to fluid accumulating around the implant. This occurs in 80% of BIA-ALCL cases.
- The lymphoma develops around the breast implant in the fluid and in most cases is contained within the fibrous capsule the body makes around the implant and is not in the breast tissue itself.
- Rarely, ALCL can develop as late-presenting capsular contracture. Two cases have recently been described with this presentation.
What is the risk of developing BIA-ALCL?
- The current risk is estimated to be 1 in 30,000. However, there has never been a documented case in a patient with only a smooth implant. We only use smooth breast implants in my office.
- Because this is a rare disease it is difficult to be certain about the absolute risk of developing this disease.
- The risk of breast cancer (in general) is about one in eight women, with or without breast implants.
Are some women more at risk than others?
- It is not possible to predict who will develop BIA-ALCL.
- It has occurred in women who have breast implants for cosmetic reasons and also for breast reconstruction.
- It has occurred in women with both saline implants and silicone implants
How is BIA-ALCL diagnosed?
- If a woman develops swelling of the breast which has an implant they are sent for an ultrasound scan and if fluid is detected this will be removed and tested for BIA-ALCL.
- Specific tests are asked for to exclude or diagnose BIA-ALCL (cytology and CD30).
- Most fluid collections around breast implants are not BIA-ALCL but proper testing will be able to tell them apart.
- Mammograms are not useful in diagnosing BIA-ALCL.
- In confirmed cases MRI and PET/CT scans may be performed to help stage the disease.
Should women with breast implants be screened for BIA-ALCL?
- At this point expert opinion is that women without symptoms or changes in their breasts do not need a regular ultrasound scan.
- Breast implants are not lifelong devices. If there are changes in your breasts associated with breast implants and especially if there is general swelling or a lump you should have a breast examination and this may need to be investigated further
What is the treatment of BIA-ALCL?
- The majority of cases are cured with the removal of implants and the fibrous capsule around them from both breasts.
- The majority of patients require no additional treatment.
- All known cases where there were no delays in diagnosis have resulted in 100% cure at this time.
Should breast implants be removed just in case?
- Breast implants are not lifelong devices and in general all will need to be removed or replaced at some point in a woman’s lifetime.
- The most common reasons for implant removal or replacement are capsular contracture, implant migration, implant rupture.
- Without symptoms or signs of BIA-ALCL routine implant removal is not required unless there are other concerns.
Do we know what causes BIA-ALCL?
- Bacteria have been identified within the lymphoma and around implants in affected breasts.
- There is accumulating evidence that a long-term inflammatory response to the presence of these bacteria is one of the factors that may cause of BIA-ALCL.
- There may also be genetic factors involved for individual women.
- We are continuing to investigate this disease to improve our understanding.
Are there ways to make breast implant surgery safer?
- There is accumulating evidence that bacteria are associated with other complications of breast implant surgery as well such as the risk for capsular contracture which does not lead to cancer.
- Infection control standards are extremely important in breast surgery to ensure best outcomes and Specialist Plastic Surgeons are expertly trained to ensure the highest standards of patient safety and lowest risk of infection.
- If you have swelling of the breast associated with a breast implant you may need a referral for an ultrasound to remove some fluid for testing and this will be able to determine if BIA-ALCL is present (Immunohistochemistry is performed to identify a T-cell lymphoma that is positive for the CD30, and cytology).
- Most breast swelling that occurs after breast implants is not due to BIA-ALCL however it does needs to be excluded.
Can new breast implants be inserted when BIA-ALCL is treated?
- Yes. Smooth implant have been replace at a delayed interval in treated patients as well as immediately in some cases.
Where can I get more information?


While about 80% of new BIA-ALCL patients may present with the classic late seroma, the lack of a seroma does not rule out the disease. There have been a small percentage of cases of BIA-ALCL that initially present with capture contracture as described above. Surgeons should be particularly aware of this possibility when seeing a patient with a late capsular contracture – especially in a patient with textured breast implants, associated fluid collection or any gross capsular abnormalities. The point of these case reviews is to remind surgeons to carefully evaluate these patients as BIA-ALCL, although very rarely, may be associated with the capsular contracture. If a surgeon’s index of suspicion preoperatively that the capsular contracture may represent something more than a typical capsular contracture, a PET scan may be indicated; however, the advisability for PET scanning routine capsular contractures is not indicated.
Current Incidence of BIA-ALCL as of February 2020 (Source: PROFILE American Society of Plastic Surgeons – https://www.thepsf.org/
 In 2017, the media caught on to a story (that is not really new) about an extremely rare condition associated with breast implants known as breast implant-associated (BIA)-ALCL.
In 2017, the media caught on to a story (that is not really new) about an extremely rare condition associated with breast implants known as breast implant-associated (BIA)-ALCL.
If you have a breast augmentation or implant-based breast cancer reconstruction, DON’T PANIC!
Some quick facts about BIA-ALCL:
- BIA-ALCL is not a breast cancer, but a rare and treatable T-cell lymphoma that usually develops as a fluid swelling around breast implants.
- The lifetime risk for this disease appears to be about 1:3,817 to 1:30,000 for textured breast implants. This equates to a 0.003 percent risk. Thus far, there have been no confirmed cases of BIA-ALCL in women who have had only “smooth-surface” breast implants.
- The FDA is not recommending removal of textured implants. Rather, the FDA recommends, as do I, that every woman conduct regular self-examination. If you develop swelling or a lump in your breast, contact my office right away. I will comprehensively evaluate you and order the appropriate tests to determine if any treatment is indicated.
- Women who develop BIA-ALCL can often be cured by simply removing the implant and the scar tissue surrounding it. Some patients may require additional treatment (such as radiation or chemotherapy). Following removal, replacement with a smooth surface implant may be an option.
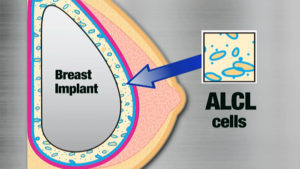
 ALCL is in the family of other lymphomas, and is a very rare subtype. BIA-ALCL is very different from systemic ALCL: this is a different disease with a very different disease progression.
ALCL is in the family of other lymphomas, and is a very rare subtype. BIA-ALCL is very different from systemic ALCL: this is a different disease with a very different disease progression.The FDA has been following extremely rare incidences of BIA-ALCL (breast implant associated anaplastic large cell lymphoma), which are nearly 100% associated with TEXTURED breast implants.
This is one of several reasons why I only use smooth silicone gel implants.
Definitive treatment of BIA-ALCL is removal of the implant and total capsulectomy (removal of the entire scar tissue capsule around the implant). BIA-ALCL associated with breast implants seem to be less aggressive than systemic ALCL. Capsulectomy and explantation usually is the only treatment needed.

Nearly ALL cases of ALCL have been associated with TEXTURED breast implants, which are not used in my Plastic Surgery practice.
Most Plastic Surgeons have never seen a case of BIA-ALCL. I have been using the #ALCL hashtag on social media for years to increase awareness of our current understanding of this rare condition. It is good that awareness is increasing about this condition so that future cases are identified and treated appropriately. This should prevent bad outcomes since it is so easily treated with surgery.
Our professional societies and research community have been following BIA-ALCL and its association clinically and articles published about this topic are available here on the PRS journal website.
Learn more from our Plastic and Reconstructive Surgery (PRS) Journal editor, Dr. Rod Rohrich:
The FDA has been following these rare breast implant-associated (BIA)-ALCL cases for many years:
Here is a more lengthy video with excellent information from my colleague Dr. Mark Clemens at MD Anderson Cancer Center:
While it remains difficult to determine the exact number of BIA-ALCL cases associated with breast implants, to date there have been NO confirmed smooth surface-only cases of ALCL reported.
Learn more about actual BIA-ALCL numbers in this ASPS-ASAPS publication and receive answers to frequently asked questions about breast implants and BIA-ALCL.
For more information, please visit the American Society of Plastic Surgeons’ web page on ALCL and the FDA’s page on ALCL.
If you have concerns about your implant, please call our office at 415-923-3067 and make a follow up appointment with Dr. Horton at your convenience.
_____________________________________________________________________________________________________

The following is the latest update about BIA- ALCL from the American Society for Aesthetic Plastic Surgery (ASAPS), published July 25, 2019:
Q: What is BIA-ALCL?
A: BIA-ALCL (Breast Implant-Associated Anaplastic Large Cell Lymphoma) is a rare spectrum of disease that can range from an indolent accumulation of fluid around the breast (seroma) to a potentially metastatic lymphoma especially when there are delays in diagnosis. BIA-ALCL is not a cancer of the breast tissue itself. When diagnosed early, it is readily curable. If the disease is advanced, chemotherapy or radiation may be required.
BIA-ALCL is currently classified as a lymphoma. Many experts believe that it behaves clinically as a lymphoproliferative disorder (LPD) that encompasses the spectrum of disease from benign CD30+ seromas, to CD30+ malignant seromas, to invasive capsular disease, and finally metastatic disease. Current ASERF research is underway to further understand the proper classification of this disorder. Similar to LPDs, BIA-ALCL is a highly treatable disease with high cure rates.
Q. Have there been any deaths due to BIA-ALCL?
A. As of September 2024, FDA reports that there have been 33 confirmed deaths globally, (13 in the United States), attributed to BIA- ALCL since the disease was first reported nearly 20 years ago.
Q: What are the symptoms of BIA-ALCL?
A: The first symptom of BIA-ALCL is usually a swelling of the breast between 2 to 28 years after the insertion of breast implants, with an average of about 8 years after implantation. The swelling is due to a collection of fluid surrounding the implant. This fluid can cause the breast to enlarge significantly over a period of days or weeks. It can also present as a lump in the breast or armpit, firmness of the breast, or pain. It is usually easily and completely treated if patients see their doctor at the first symptom.
Q: What is the risk of developing BIA-ALCL?
A: The FDA reports that it is 1:3,817 to 1:30,000 in their latest statement. These risk assessments are changing on an ongoing basis, but this is the most accurate information currently available.
Based on current data, the risk can be further defined by the degree/type of texture of the implants as follows:
• Grade 1 (Smooth only) – In global databases, there has not been a confirmed case of BIA-ALCL in a patient who had smooth implants only. In the Feb 2019 FDA statement, it was reported that “there have been reports of BIA-ALCL in patients with smooth surfaced implants and many reports do not include the surface texture of the implant at the time of diagnosis.” At the time of this publication it is known that a single case of smooth only BIA-ALCL was originally reported to the FDA; however, it was later determined that this was not accurate and that the patient had received textured implants followed by smooth implants, and the report to the FDA was amended.
• Grade 2 (e.g. Microtexture, Siltex and similar) – 1:82,000
• Grade 3 (e.g. Macrotexture, Biocell and similar) – 1:3,200
• Grade 4 (e.g. Polyurethane) – 1:2,800*
*Based on data from an Australian study – however this was 100% Silimed polyurethane implants that had a manufacturing defect and have since been taken off the market.
Loch-Wilkinson, A., et al. (2017). “Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk.” Plast Reconstr Surg 140(4): 645-654
On July 24, 2019, in response to a request by the FDA, Allergan reported a voluntary recall of all BIOCELL textured implants and expanders, worldwide. The recall includes the Natrelle Saline-Filled breast implants, Natrelle Silicone-Filled breast implants, Natrelle Inspira SiliconeFilled breast implants, and Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled breast implants.
The recall also includes tissue expanders used by patients prior to breast augmentation or reconstruction, including Natrelle 133 Plus Tissue Expander and Natrelle 133 Tissue Expander with Suture Tabs.
“Based on the currently available information, including the newly submitted data, our analysis demonstrates that the risk of BIA-ALCL with Allergan BIOCELL textured implants is approximately six times the risk of BIA-ALCL with textured implants from other manufacturers marketing in the US,” the FDA said in a statement.
Q: What does FDA mean by a “recall”? Should current BIOCELL patients be contacted and have their implants removed?
A: This is a recall of unused Biocell implants (Biocell inventory and sold Biocell products) but not Biocell implants that have been used in patients who are asymptomatic. The FDA does not recommend or suggest that asymptomatic patients be explanted; rather that that the company refrain from selling BIOCELL implants moving forward.
Q: If a breast implant patient sees a plastic surgeon when she develops a first symptom, will she be cured?
A: That answer is not known and is a very important piece of information for patients and plastic surgeons. Most of the time patients see their plastic surgeon right away when they develop enlargement of the breast. In these cases, the disease is almost always cured with a straightforward operation. Some women with advanced disease did not act on earlier symptoms or saw a doctor who did not properly diagnose/treat them. There are a few patients who presented with advanced disease who said that they never had earlier symptoms.
Q: Can you explain the differences in implant texture and what role that factor plays in the research?
A: BIA-ALCL appears to currently develop exclusively in women with textured implants. To date there has not been a case of BIA-ALCL in a patient with only smooth implants. There are several theories which attempt to explain the higher rate for textured implant patients: one theory is that the increased surface area of textured implants allows a higher number of bacteria around the implant, which forms a biofilm in some patients, and can result in chronic inflammation, ultimately leading to a proliferation of lymphocytes. Another theory is that textured implants create greater inflammation because of chronic mechanical irritation, and another postulates that microscopic shedding of silicone from the textured wall induces inflammation.
Q: Is it a problem with Saline or Silicone implants?
A: Of the 457 unique cases of BIA-ALCL (FDA 2-2019) implants are both silicone and saline. It appears to purely be related to the surface of the implant and not to what the implant contains.
Q: How does this impact those with breast implants?
A: ASAPS and ASERF emphasize that the most important issue for all women, with and without breast implants, is to screen for breast cancer with self-exam, a regular physician exam, and mammography/ultrasound/MRI as recommended by their physician. Since breast cancer occurs in approximately 1 in 8 women, breast cancer is a far greater risk than BIA-ALCL. All women should see their plastic surgeon immediately if they note a change to the size, feel, or shape of their breasts.
Q: What about those considering breast implants?
A: Patients considering breast implants should discuss the issue with their plastic surgeon. Since our knowledge of this condition is continuing to evolve, thanks in large part to ASERF-sponsored research, patients should check surgery.org and the FDA website for any updates.
Q: What if a doctor is recommending textured implants to a patient?
A: The choice of implant type is ultimately a decision between an educated patient and her board-certified plastic surgeon.
Q: How is BIA-ALCL diagnosed?
A: If a woman develops swelling in an augmented breast, she should undergo an ultrasound scan. If fluid is detected, it should be drained and tested for:
1. Cytology
2. CD30 cell marker
CD30 immunohistochemistry is not diagnostic for BIA-ALCL; however, it is a marker for activated T-Cells. If a patient’s seroma is CD30 positive, and the cytology is negative, this likely represents a precursor to BIA-ALCL, and should be treated with total capsulectomy. If the seroma test is CD30 negative with negative cytology, then it should be treated as a benign seroma using the individual surgeon’s protocol.
The majority of seromas seen clinically are benign seromas and not BIA-ALCL. Management of all seromas should be by a board-certified plastic surgeon. Mammograms are not useful in diagnosing BIA-ALCL. In confirmed cases PET or MRI/CT scans may be used to help stage the disease.
If a patient wants to have their textured implants removed and replaced, the options are:
• Exchange to smooth implants
• Exchange to smooth implants with a capsulectomy
Q. How is BIA-ALCL treated and what is the prognosis?
A. Current recommendations for the treatment of BIA-ALCL call for bilateral capsulectomy (removing all the scar tissue) and removal of the old breast implants. This is a very common procedure performed by plastic surgeons, identical to what is done when an implant has ruptured or capsular contracture has developed. Smooth implants can be inserted or the patient can choose not to have implants replaced. In all early stage cases, the disease has been fully cured by this surgery alone. The majority of patients require no additional treatment. However, if the disease has spread to lymph nodes or grown into the adjacent tissues, chemotherapy and radiation may be necessary.
Q: Are some patients at greater risk than others?
A: The rates of BIA-ALCL seem to have different rates throughout the world. This may be due to different reporting and registries, but there is likely to be a genetic predisposition that is not yet fully understood. For instance, as of this time there are very few cases in Asian patients. As mentioned, the risk is only with textured implants and not smooth implants; the rate is no different between silicone and saline; it occurs in both cosmetic and reconstructive patients. There is no test to determine whether one textured implant patient is at any more risk of developing this disorder than any other patients.
Q: Should patients have their implants removed because of BIA-ALCL?
A: For textured implant patients, neither the FDA nor any plastic surgery society currently recommends that women should preventatively remove textured breast implants to prevent BIA-ALCL. However, there are women who have been concerned enough about BIA-ALCL and have chosen to have their implants removed. There are some women who were already considering a breast implant revision, and the BIA-ALCL issue gave them one more reason to proceed.
Breast implant patients should have ongoing follow up. Current FDA recommendations and ASAPS recommendations are that patients with textured implants with no symptoms should not do anything and implant removal is not recommended.
Q: Should women with breast implants be screened for BIA-ALCL?
A: There is no blood test to specifically screen for BIA-ALCL. The expert opinion is that asymptomatic women without breast changes do not require more than routine mammograms and breast exams. But if a patient experiences a change in her breasts – especially if there is swelling or a lump – she should undergo immediate examination, imaging, and consultation with a plastic surgeon. If there is fluid around the implant the fluid should be aspirated under ultrasound guidance and sent for analysis.
Q: What causes BIA-ALCL?
A: ASAPS, ASERF, the FDA, and the implant manufacturers are intensely studying BIA-ALCL. To date, no specific causal factors have been identified. Implant texturing, bacteriologic contamination, and genetic factors have been implicated and are undergoing further study.
The best theory today is that a combination of factors are required for the development of BIA-ALCL:
1. Textured implants (surface area to sequester bacteria)
2. Chronic inflammation
3. Genetic predisposition
4. Time
Genetic factors may play a role. Some geographic areas have reported very few cases. Ongoing data collection worldwide will help to determine whether there are any genetic propensities for this disease.
Q: Does ASAPS recommend against the use of textured implants?
A: The best practice is for the physician to discuss with each patient the known risks and potential complications associated with any procedure.
Q. What is the recommended clinical response to a patient presenting with symptoms that could be attributable to ALCL?
A. Detailed information can be found on the ASAPS website at: http://www.surgery.org/professionals
Q: Where can I find more information on BIA-ALCL?
A: Additional information and resources on BIA-ALCL are available online at https://www.surgery.org/media/resources
Q: Is there any assistance available to the patient?
A: The Mollenkopf Aesthetic Breast Reconstruction Fund and the BIA-ALCL Patient Assistance Fund can offer financial assistance to patients. Additionally, Sientra offers to cover lab testing for any seromas associated with their implants.
Q: What research is being conducted?
A: ASERF is currently funding two BIA-ALCL studies on the Pathogenesis of BIA-ALCL and Genomic Profiling to Understand the Pathogenesis of BIA-ALCL. ASERF is sponsoring leading, cutting edge research on BIA-ALCL to better define the disease and improve diagnosis and outcome. More information can be found on the ASERF website: www.aserf.org

